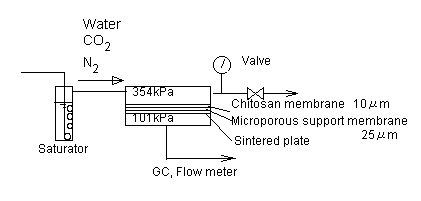
 |
| Figure 1 The measurement apparatus for a mixed gas permeation through a wet chitosan membrane. |
Die Angewadndte Makromolekulare Chemie 248 (1997) 85-94 (Nr. 4329)
Department of Material and Chemical Engineering, Niigata University, Niigata 950-21, Japan
Permeability of CO2 through chitosan membrane swollen by water vapor in feed gas
Akira Ito*, Makoto Sato, Tomotoshi Anma
SUMMARY:
The permeation and separation of carbon dioxide through a water-swollen chitosan
membrane was studied. A chitosan membrane exhibited a large gas permeability
when it was swollen by water vapor contained in the feed gas. Carbon dioxide
preferentially permeated through the swollen chitosan membrane with a
permeability of 2.5×10-8cm3(STP)・cm/(s・cm2・cmHg)
and a CO2/N2 separation factor of 70 at room temperature. This separation
performance for CO2 resulted from a basic property of the amino groups in the
chitosan molecules. The membrane preparation method such as acetic acid
concentration of the casting solution affected the membrane permeation rate. The
effect of operation temperature was also measured. To increase the separation
performance of the membrane, several methods of membrane treatment and operation
were evaluated.
Introduction
Chitin is the second largest biopolymer resource after cellulose. Chitosan is obtained by the deacetylation of chitin. New applications of chitosan to separation processes, such as absorption or membrane separation, will make a contribution to further utilize these resources. The chitosan macromolecule has unique properties because of the presence of both amino and hydroxy groups in it. This chemical structure provides two features to chitosan. The first is a hydrophliic property, which gives solvent stability and a swelling property by water. The second is a basic property, so that chitosan is easily dissolved in aqueous acetic acid of low concentration.
In the research field of membrane separation, the chitosan membrane has been mainly applied to the pervaporation process. Many studies have reported that the chitosan membranes were highly water-permselective for the pervaporation of aqueous alcoholic solutions. Ohya et al. [1,2] reported on this process and discussed their results from the view point of the swelling properties of the membrane. Uragami et al. [3-5] or Lee[6] applied chitosan derivative membranes to the pervaporation of an ethanol-water mixture so that they improved the dehydration performance of the process. For vapor state permeation or evapomeation, chitosan derivative membranes, such as carboxymethyl chitosan, also exhibits a preferential permeation of water vapor over ethanol, which was reported by Goto[7] and Uragami[3]. We can restate that these studies of the pervaporation processes utilize the hydrophilic property of chitosan.
On the other hand, only a few reports[8] have dealt with the permeation of gases, including carbon dioxide, through the chitosan membrane. The reason for this is that the chitosan membrane has a low permeability for gases in its dry state. For a hydrophilic polymer membrane, a low permeability of gases through it could be much improved if it is used in a wet state by water. A feed saturated water vapor will make hydrophilic membrane in a swollen state and increases permeability. By use of a water-vapor containing feed, a permeability increase in O2 and N2 was shown for a starch membrane[9] or a polyvinylalcohol membrane[10]. Similarly, a hydrophilic chitosan membrane can be expected to have an increased permeability with a water-saturated feed. Furthermore, with the presence of water in the polymer, the basic property of chitosan may exhibit a preferential permeation for an acid gas such as CO2.
The object of this report is to study gas permeation process through a wet chitosan membrane from the view point of CO2 separation. Permeation experiments were conducted using a CO2/N2 mixed gas which was saturated by water vapor. The effects of membrane preparation conditions and operating conditions on the separation performance of the wet chitosan membrane are reported.
Experimental
Membrane preparation
Chitosan with a nominal degree of deacetylation of 70% was purchased from Katokichi Co. Ltd., Japan. Chitosan was dissolved in a aqueous acetic acid solution at a concentration of 2 wt%. The acetic acid concentration of the solution was normally 4 wt%. The solutions were cast on a hydrophilic-treated polypropylene microporous membrane of 40% porosity and 25μm thickness [Celgard 3501 (Celanese Separation Products, U. S. A.)]. The evaporation of water and acetic acid was allowed to occur under ambient conditions. The membranes were then dried for 1 day in a 50C oven. The thickness of the chitosan dense layers were 7~17μm.
Permeation measurement
The experimental setup is shown in Figure 1. The apparatus consisted of a flat-type membrane cell with a 19cm2 permeation area, a gas flow system which delivered a CO2 /N2 mixed gas, a water-vapor saturator and flow and concentration measurement equipment. Permeation rates were measured by volume displacement using soap bubble meters. Gas samples were analyzed by gas chromatography. The upstream and downstream pressures were maintained at 354kPa and atmospheric pressure, respectively.
 |
| Figure 1 The measurement apparatus for a mixed gas permeation through a wet chitosan membrane. |
The permeation rate of a dry chitosan membrane was too low to measure. The permeation rate became measurable and reached a steady state after a 50-hour run from the setting of a dry membrane.
Results and Discussion
Effect of humidity in the feed on permeation rate
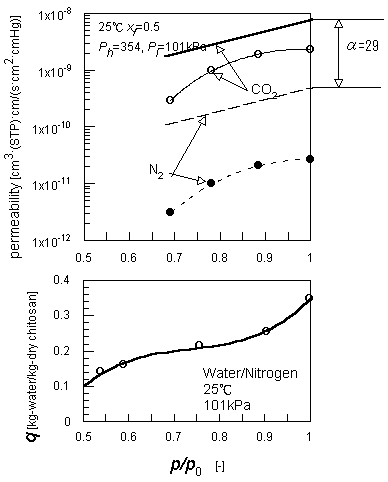 |
| Figure 2 Relation between permeabilities through a wet chitosan membrane and humidity of feed gas. |
The permeability of gases through a wet chitosan membrane was controlled by the humidity of the feed. Figure 2 shows the permeation results of the humidified CO2 or N2 gas. The permeability data are plotted versus the humidity , p/p0, of the feed gas, where p0 is the saturated vapor pressure of water and p is the water vapor pressure in the feed. The partial pressure of the water vapor in the feed was changed by cooling of the saturator and was estimated from the temperature of the saturator. The humidity of the feed gas significantly affects the permeability of the gases.
At the bottom of the figure, the sorption equilibrium of chitosan for water vapor is shown. It was measured from the weight gain of a chitosan film in an atmosphere of controlled humidity[11]. The upper range of the S-shaped isotherm (p/p0>0.75) means the content of free-water in the swollen membrane. The permeation rate of gases was able to be measured over this humidity range of the feed. The free-water contained in the polymer matrix would be the permeation path of the gases.
The permeation rete of gases through a swollen or gel membrane can be estimated from the diffusion coefficient through it, D, and henry constant of water, H. Paul[12] derived a generalized relation between swelling ratio, q, and D/D∞, where D∞ is the diffusion coefficient of a gas in pure water. Assuming this relation is applicable to the wet chitosan membrane, the permeation flux though it, N[mol/(m2.s)] , can be calculated as follows:
N=CD(Ph/H-Pl/H)/ δ(1)
where C [mol/cm3] is the molar density of the diffusion medium or water in the polymer, Ph is the feed pressure, Pl is the permeate pressure and δ is the membrane thickness. Estimated results are shown in the figure in terms of permeability ( P=Nδ/( Ph - Pl)). Because of the simplicity of the estimation, the absolute values of the results may have no significance. But, it is essential that a pseudo water layer has an ideal separation factor of 28.5 for CO2 over N2. This value mainly depends on the henry constants of these gases over water. On the contrary, the measured separation factor of 70 is much larger than that of the pseudo water layer. This suggests the participation of chtosan's basic property in the preferential permeation of the acid gas, CO2.
Permeation of a mixed gas
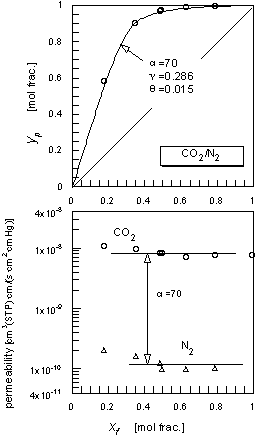 |
| Figure 3 The CO2/N2 mixed gas permeation results. |
Figure 3 is the CO2/N2 mixed gas permeation results of the chitosan membrane swollen by the saturated water vapor in the feed at room temperature. The permeate concentrations, yp, are shown versus the feed gas concentrations, xf. The permeabilities of individual components were evaluated based on the partial pressure differences and the membrane thickness of the dry base. The permeabilities did not show a concentration dependency and the separation factor, α, of 70 was constant at the various feed gas compositions. The literature on CO2 membrane separation using facilitated transport phenomena reported the dependency of CO2 permeability on the partial pressure especially at a low concentration range13,14. In this study, there was no indication of facilitated transport of the acid gas. The solid line in the top figure is the relation between xf and yp forα=70 assuming the perfect mixing condition of both the feed and permeate sides15, where experimental conditions were used for the pressure ratio, , and cut, .
The permeation results for the CO2/CH4 mixed gas are shown in Figure 4. The separation factor of the system was about 28.
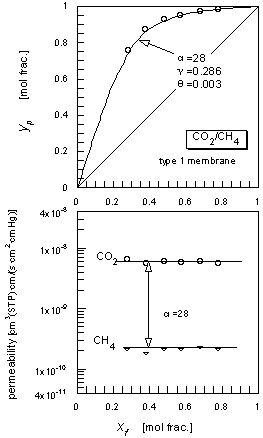 |
| Figure 4 The CO2/CH4 mixed gas permeation results. |
The effect of operating temperature on the process was measured using a setup where the apparatus with the saturator was placed in an air-bath thermostated at the desired temperature. The results are shown in Figure 5. Generally, there are two effects of temperature on the permeation rate through a swollen membrane. One is that the permeability or diffusion coefficient through a polymer increases with temperature. The other is that the adsorbed water content in the polymer decreases with temperature.
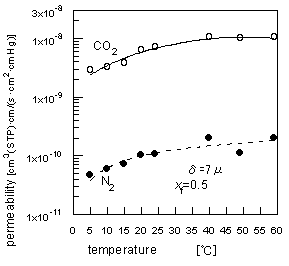 |
| Figure 5 The effect of operation temperature on the permeabilities for CO2/ N2 mixed gas permeation. |
The observed trend was due to these opposite effects. Operation at a high temperature has a small advantage in the present process.
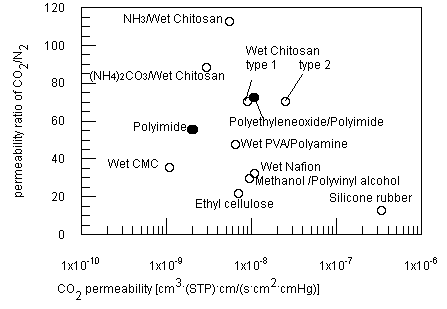 |
| Figure 6 Comparison of separation performance of membranes for CO2/ N2 mixed gas. Numbers denote reference numbers. |
Figure 6 shows the CO2 separation performance of chitosan membranes and other
polymer materials at room temperature. The ideal separation factors of membrane
are plotted versus permeabilities of CO2. As shown in the figure, several
additional methods have been effective to increase the separation performance of
the original chitosan membrane as follows:
(1) The permeation rate and separation factor increased using a thin-layer
coating (<3μm ) of carboxymethyl cellulose on the surface of the
chitosan membrane. Carboxymethyl cellulose is one of the most water-absorbable
or high hygroscopic polymers. The improvement of separation by the treatment
could be derived by increasing of the swelling ratio of the membrane.
(2) If we can introduce a trace amount of ammonia gas into the feed flow,
the CO2 separation factor of the wet chitosan membrane will increase. A
permeation run was conducted using 2% ammonia-water in the saturator. The result
of an increased separation factor is shown in the figure.
(3) The permeability of the chitosan membrane is affected by the membrane
preparation conditions. Among the preparation conditions, the concentration of
acetic acid in the cast solution had a large effect on the permeation rate. The
compositions of the solvents were changed from 4% to 83% acetic acid. Using the
cast solution with a high concentration of acetic acid , we could increase the
permeation rate of a chitosan membrane by a factor of 2.5. The effect of acetic
acid concentration in the cast solution on the permeation rate may be caused by
the forming of a semi-microporous structure during the evaporation process of
water and acetic acid.
Figure 6 shows the comparison of this work and other membranes for the CO2 separation. Polyimide derivatives are most attractive membrane material among the solid polymers[16]. Facilitated transport membranes, such as an amine-containing or grafted membrane, have the highest separation factors and permeation rates[17,18]. One drawback of the facilitated transport membranes is that these are usually tested and operated in a sweep gas permeation mode. The present wet chitosan membrane has a comparable separation performance to these membranes.
Conclusion
The application of a biopolymer for CO2 separation was studied. The wet chitosan membrane had larger permeabilities and higher separation factors than solid polymer membranes. The separation factor of the process was derived by the interaction between the acid gas, CO2, and the base-polymer, chitosan. The high permeation rare was caused by the swollen state of the polymer. The advantages of the present process are the reuse of the biopolymer resource and the utilize of water vapor in a flue gas as a separation agent of CO2.
references
1 G. Qunhui, H. Ohya, Y. Negishi, J. Membrane Sci. 98 (1995) 223
2 H. Ohya, Q. Guo, Y. Negishi, Water. Treat. 9 (1994) 235
3 T. Uragami, H. Kinoshlta, H. Okuno, Angew. Mnkromol. Chem. 209 (1993) 41
4 T. Uragami, H. Shinomiya, J. Membrane Sci. 74 (1992) 183
5 T. Uragami, Desalination 90 (1993) 325
6 Y. M. Lee, E. M. Shin, Angew. Makromol. Chem. 192 (1991) 169
7 M. Goto, A. Shiosaki, T. Hirose, Sep. Sci. Technol. 29 (1994) 1915
8 R.-K. Bai, M.-Y. Huang, Y.-Y. Jiang, Polym. Bull. 20 (1988) 83
9 R. Sala, I. Tomka, Angew. Makromol. Chem. 204 (1993) 161
10 W.-Z. Zhang, M. Satoh, J. Komiyama, J. Membrane Sci. 40 (1989) 342
11 J. Y. Lee, P. J. Westgate, M. R. Landisch, AIChE Journal 37 (1991) 1187
12 D. R. Paul, Sep. Purif. Methods 5 (1976) 33
13 J. D. Way, R. D. Noble, D. L. Reed, G. M. Ginley, L. A. Jarr, AIChE Journal
33 (1987) 480
14 H. Matsuyama, M. Teramoto, K. Iwai, J. Membrane Sci. 93 (1994) 237
15 E. Kesting, A. K. Fritzsche, Polymeric Gas Separation Membranes, Jhon Wiley
& Sons, INC., 1993, p. 342
16 K. Okamoto, N. Umeo, S. Okamyo, K. Tanaka, H. Kita, CHEMISTRY LETTERS (1993)
225
17 H. Matsuyama, M. Teramoto, H. Sakakura, J. Membrane Sci., 114 (1996) 192
18 D. Langevin, M. Pinoche, E. Selegny, M. Metayer, R. Roux, J. Membrane Sci. 82
(1993) 51